Is the inability to observe electrical excitation and mechanical contraction simultaneously limiting your toxicological risk assessment?


For years, cardiac safety pharmacology faced a structural mismatch between physiological relevance and screening capacity. Researchers relying on manual patch clamp achieved high electrical fidelity but lacked the throughput for industrial screening. Conversely, automated high-throughput systems often relied on overexpressing cell lines lacking physiological complexity.
The critical engineering bottleneck was the inability to perform functional cardiac phenotyping that captured both electrical signaling and mechanical beating in the same well, at the same time. This separation created ambiguous phenotypes, where compounds might perturb signaling and contraction differently (electromechanical dissociation). Without a unified readout, identifying drug-induced arrhythmia required triangulating data from disjointed assays, forcing expensive manual follow-up and undermining the efficiency of high-throughput screening.
To support Nanion Technologies in industrializing cardiac safety workflows, Sciospec engineered a modular measurement core capable of simultaneous impedance and field potential recording. Scaling to industrial throughput creates a massive data bottleneck. Transmitting raw data from 96+ wells simultaneously can crash standard data interfaces. The architectural challenge was not merely sensing, but tight and close to sensor integration of data acquisition, stimulation and signal extraction. Treating demodulation and impedance extraction as a downstream software task would fail under the bandwidth constraints of 96 well cardiac assay iPSC cardiomyocytes. Sciospec addressed this by embedding signal processing directly at the acquisition point. The system utilizes fully parallel real-time impedance demodulation 96 well architectures to reduce large time-domain data streams into information-rich representations before transmission. This on-board processing enables phase-accurate synchronization of excitation-contraction coupling measurement without multiplexing artifacts. By decoupling the measurement core from the interface, the architecture preserved signal integrity at millisecond resolution, enabling the rigorous detection of rapid arrhythmic events.
Success in industrializing biology requires more than off-the-shelf hardware. It demands a collaborative engineering process where biological constraints drive architectural decisions. By establishing a modular core, the partnership minimized the technical risk associated with scaling from single-plate validation to high-volume industrial workflows, ensuring that validation data remained consistent as channel counts increased.
Nanion was Sciospec´s earliest OEM partner and successfully leveraged our technical capabilities to create high impact products that continue to shape the field.
The partnership continues to create new products and solutions to keep up with needs of industry and research. We are proud to be part of this and look forward to many more exciting joint developments.
From Qualitative Trends to Absolute Metrics
The new approach doesn´t just monitor trends – it quantifies them and it eliminates “Electromechanical Dissociation”: Simultaneous recording detects compounds that alter electrical signaling without affecting the beat (or vice versa), preventing false negatives common in disjointed assays. By aligning workflows with CiPA-oriented functional assessment, laboratories can fail unsafe compounds earlier based on integrated functional risk rather than surrogate markers alone.
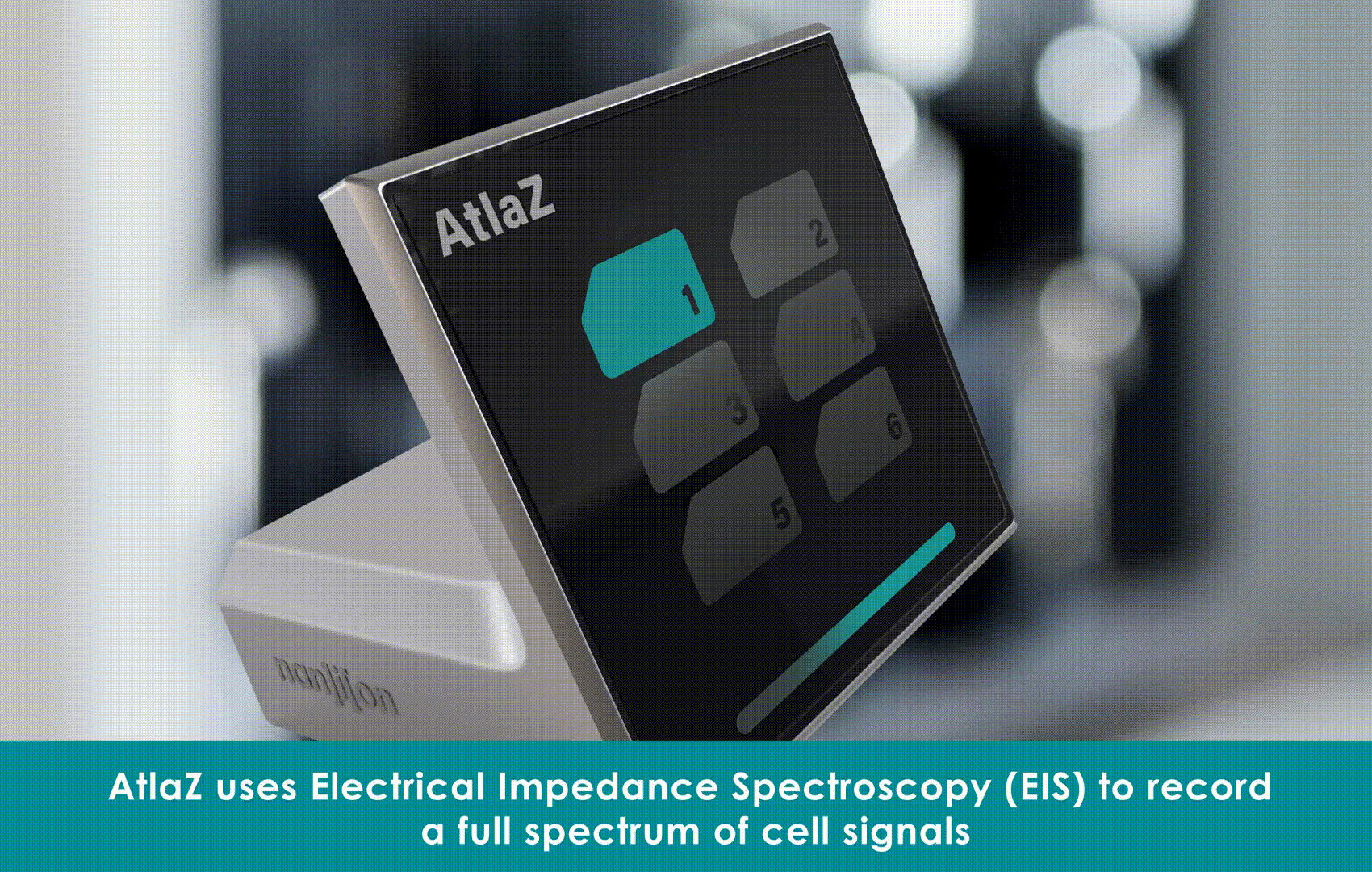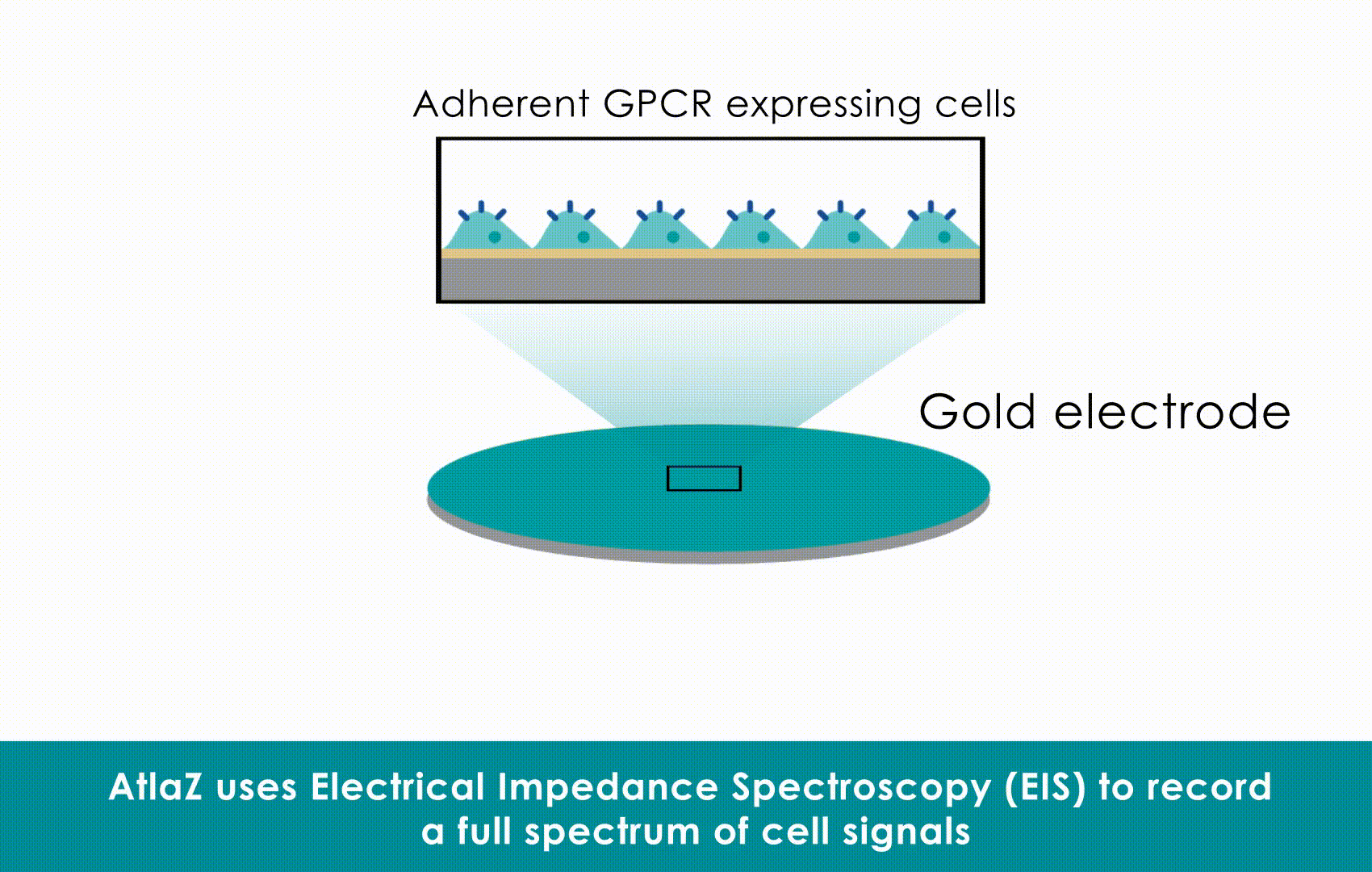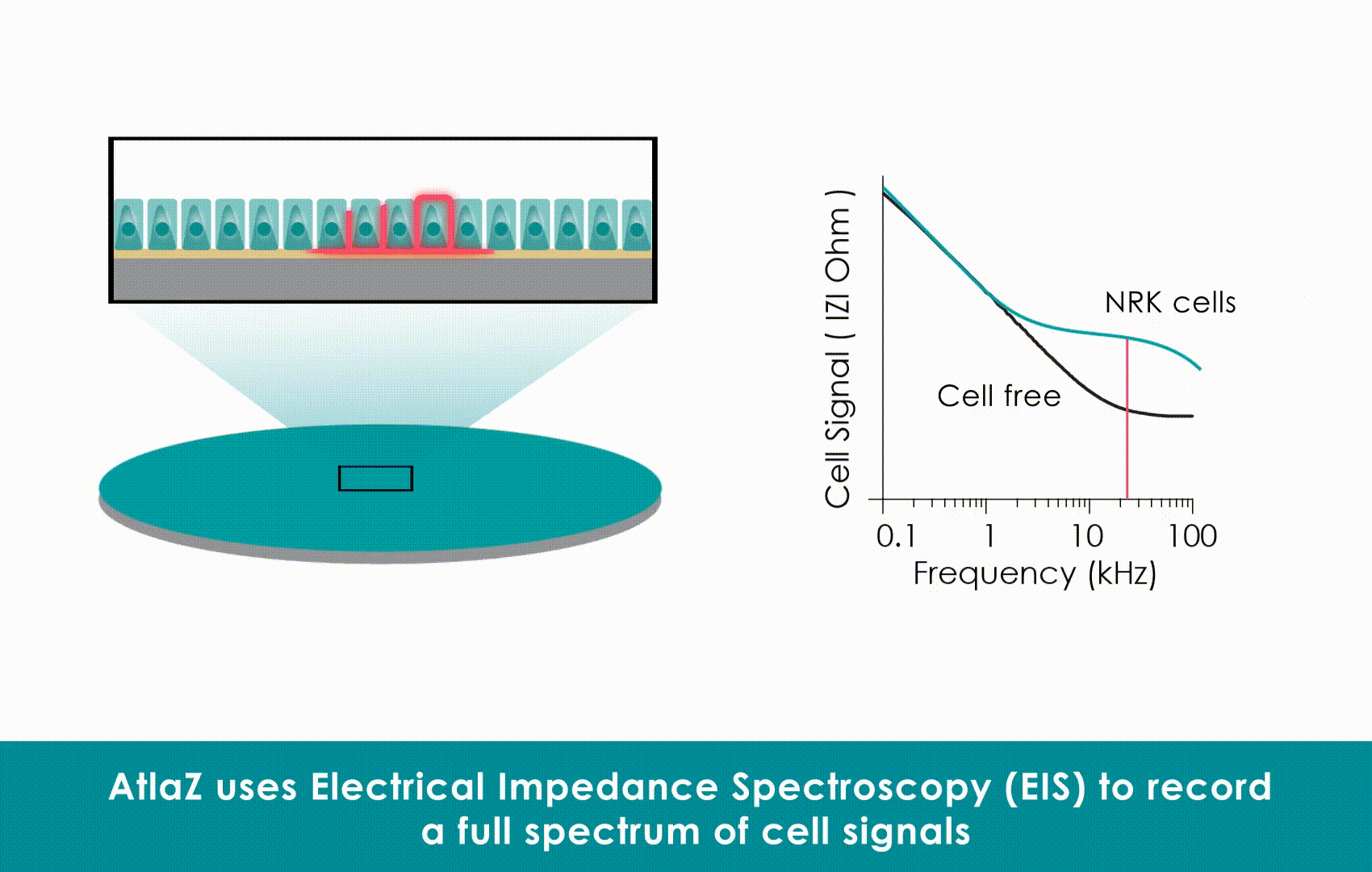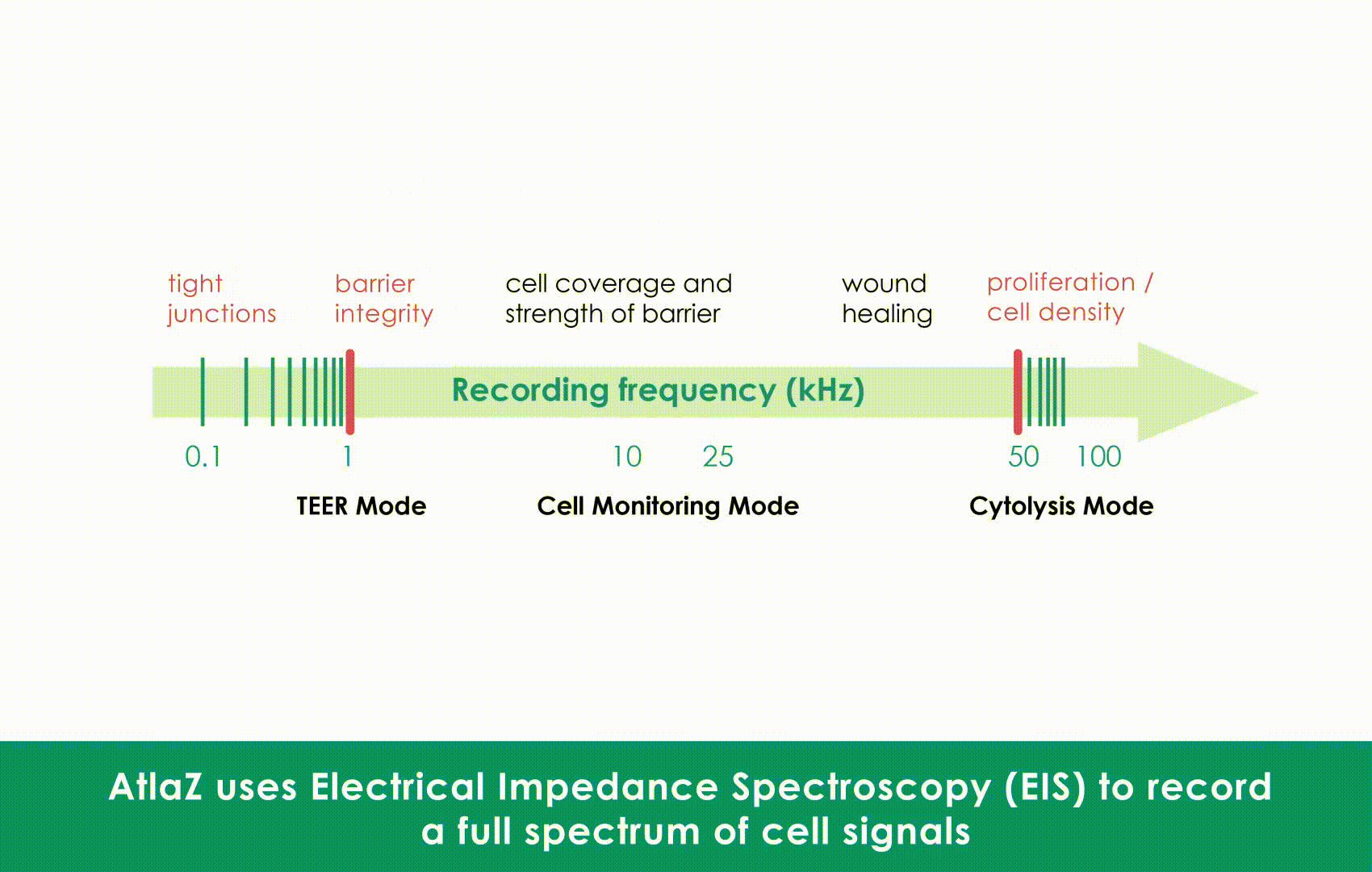
Critically, this high-fidelity impedance data allows for the calculation of absolute contractile stress. Research leveraging this measurement fidelity established a power-law relationship between impedance signals and mechanical stress (approximately 9.5 kPa), moving label-free cardiotoxicity testing beyond simple trend monitoring. This transforms impedance from a qualitative trend into a robust, reproducible metric for functional cardiac phenotyping.

The CardioExcyte 96 platform, powered by this measurement core, has been validated by over 50 peer-reviewed studies and adopted by industry leaders for CiPA framework assessment. The reliability of the hardware in detecting drug-induced arrhythmia and defining safety margins has made it a staple in laboratories ranging from Merck KGaA to the Center for Arrhythmia Research and Therapeutics at Vanderbilt University.
Prof. Bjorn C. Knollmann, Director, Center for Arrhythmia Research and Therapeutics, Vanderbilt University validated the platform’s role in their workflow:
I was particularly intrigued by the pacing feature... the dual EFP + impedance capability... For the first time, rate-dependent changes of cardiac electrophysiology and contractility can be easily quantified."
Prof. Bjorn C. Knollmann Tweet
Hans-Peter Scholz, Technician, Merck KGaA noted that “Analysis templates can be used to re-do the same analysis procedures… reducing the effort for data handling to just a couple of mouse clicks… Furthermore, multiple export options are available to allow a seamless integration in databases.” and
Prof. Dr. Joachim Wegener, University of Regensburg summed up the importance of this development:
Electrical Impedance Spectroscopy will become just as relevant for adherent cells as flow cytometry is relevant for cells in suspension
Prof. Dr. Joachim Wegener Tweet

What started with CardioExcyte did not stop at a single product generation. As assay requirements diversified and throughput, flexibility, and modality integration became more critical, the same core architecture evolved further—powering platforms such as Flexcyte and AtlaZ. Each step added new capabilities: broader assay compatibility, expanded form factors, and tighter integration into automated workflows. Importantly, this evolution did not require a reset or reinvention of the underlying technology. Instead, it reflects a partnership model where platforms grow alongside scientific needs. For Nanion, this meant extending a proven foundation rather than being constrained by it. This is exemplary for Sciospec´s approach: working with Sciospec is not a dead end or a locked-in design choice—it is a path where systems, capabilities, and ambitions scale together through long-term, productive collaboration.
Nanion required 1 ms temporal resolution and synchronized EFP to industrialize their cardiac biology. They leveraged our architecture to create the CardioExcyte 96, AtlaZ and Flexcyte replacing fragmented assays with a unified platform. Now, we invite you to do the same for your instrument concept.
Don’t let electronics be your bottleneck. What are the critical requirements for your instrument?

💡Curious for more? Check out our latest developments and products.
🧪Want to try it yourself? Contact us for more information
🚀Looking for a tailored solution? Our experts can help!
📢 Follow us on LinkedIn for more updates.
The Comprehensive In Vitro Proarrhythmia Assay (CiPA) is a regulatory framework that assesses overall proarrhythmic risk, whereas hERG testing only screens for a single potassium ion channel blockade. CiPA integrates multiple ion channel effects with in silico modeling and functional checks using human iPSC-derived cardiomyocytes to predict clinical risk more accurately.
Why it matters: The industry is moving toward this integrated risk assessment to avoid the false positives common in hERG screening. Sciospec’s architecture specifically addresses CiPA Pillar 3 (functional verification) by providing the comprehensive phenotypic data—electrophysiology and contractility—required to define safety margins beyond simple surrogate markers.
High throughput screening (HTS) is the automated process of testing thousands of chemical compounds against biological targets to rapidly identify active drugs, antibodies, or genes. In cardiac safety, valid HTS must replicate human physiology at industrial speeds, often using automated liquid handling and multi-well plate formats.
Success in “industrializing biology” requires more than just speed; it requires handling massive data loads. Sciospec’s measurement core scales from single channels to 576 parallel channels and beyond. By embedding signal processing at the sensor (Edge Computing), we solve the bandwidth bottleneck that typically presents a hard boundary for scaling to high-volume cardiac screening.
Cardiotoxicity refers to heart damage caused by drugs or chemical compounds, often manifesting as arrhythmia, structural damage, or loss of contractile force. Detection typically involves screening compounds against heart cells (cardiomyocytes) to monitor changes in beating rate, rhythm, and force.
The Shift in Detection: Traditional methods often relied on simple cell death assays or single-channel screens. However, modern industrial workflows utilize functional cardiac phenotyping—measuring the actual physiological performance of the cell. Sciospec’s architecture empowers this by detecting functional risks (like electromechanical dissociation) that surrogate markers miss.
Impedance-based assays are generally superior for high-throughput screening because they are label-free, non-invasive, and data-efficient. Unlike optical imaging, which requires dyes that can cause phototoxicity and generate massive image files, impedance offers real-time kinetic monitoring with lower data overhead and higher temporal resolution.
Technical Comparison: While optical methods struggle with focus drift and motion artifacts , Sciospec’s impedance architecture delivers 1 ms temporal resolution. Furthermore, this high-fidelity data allows for the calculation of absolute contractile stress (approx. 9.5 kPa), transforming qualitative trends into robust, reproducible metrics without the variability of image analysis.
MEA refers to Multi Electrode Arrays, so technically speaking this is type of sensor arrangement – typically with a grid of electrodes.
However, in literature MEA-measurements often refer to the measurement of the electrical activity (field potential) of cells, while impedance measures the physical shape changes (contractility) and attachment. While often used separately, they are most powerful when combined. And this can be done on MEA form factors as well – provided the right technolgy – refer to Sciospec MEArack as an example.
The Hybrid Solution: You don’t have to choose. Sciospec’s “hybrid impedance-plus-EFP” architecture records both modalities simultaneously in the same well. This provides a complete picture of the heart beat: the electrical trigger (MEA/potential measurement) and the resulting mechanical contraction (Impedance), fully synchronized at 1 ms resolution.
Human iPSC (induced pluripotent stem cell) cardiac assays are increasingly viewed as more predictive of human clinical response than animal models due to species-specific differences in ion channels. Validated iPSC platforms allow researchers to detect liabilities earlier and reduce reliance on animal testing.
Validation Data: The reliability of this specific hardware has been validated by over 50 peer-reviewed studies. A blinded study by Bot et al. (2018) demonstrated that Sciospec’s architecture yields consistent excitation-contraction coupling results across different operators and independent laboratories, meeting the rigorous reproducibility standards required for industrial adoption.
Electrophysiology refers to the electrical excitation signals (Field Potential) that trigger the heart to beat, while contractility is the resulting mechanical physical beating of the heart muscle. In drug safety, a compound can impact one without affecting the other, a phenomenon known as electromechanical dissociation.
The “Whole Picture” Solution: Measuring these independently leads to “ambiguous phenotypes”. Sciospec’s technology captures simultaneous Impedance (Contractility) and Extracellular Field Potential (EFP) in the same well. This unified readout ensures direct correlation of excitation-contraction coupling, preventing false negatives where electrical signals persist but the mechanical beat has stopped.
Label-free technology monitors cell biology without using dyes, radioactive tags, or fluorescent markers that can alter cell behavior. It uses non-invasive biosensors (like impedance electrodes) to measure cell health and function in real-time.
Why it wins for Safety: Labels can be toxic or fade over time (photobleaching). Label-free monitoring allows for “chronic” long-term observation of cells without damaging them. Sciospec’s technology takes this a step further: it is not just a trend monitor; it offers absolute contractile stress quantification (~9.5 kPa), making label-free data just as quantitative as invasive methods.
Excitation-contraction coupling (ECC) is the biological process where an electrical action potential triggers a heart muscle cell to contract. Disrupting this link is a known mechanism of drug toxicity.
The Synchronization Challenge: To measure ECC accurately, you must see the electrical spike and the mechanical twitch at the exact same moment. Sciospec’s core embeds signal processing at the acquisition point to ensure phase-accurate synchronization of these two events. This allows researchers to see exactly how a drug affects the time delay between “excitation” and “contraction”.
explore by relevant keywords
…or just browse our most recent posts: