Is optical analysis limiting the scale up of your organ on chip application?


Pharmaceutical leaders like Merck, Sanofi, and Roche are transitioning to advanced organ-on-chip models to replace low-fidelity 2D cultures. However, scaling these assays for high throughput screening presents a critical engineering conflict. Traditional optical microplate readers, while precise, impose large footprints and multi-day occupation times that block parallel experiments.
Furthermore, conventional handheld meters lack the sensitivity to resolve the real-time barrier integrity of miniaturized 3D tissues, where low absolute resistance and complex clustering make standard measurements functionally impossible.
To achieve industrial scale, researchers required a label-free, membrane-free sensing architecture capable of quantifying perfused organ-on-chip responses without the interference of destructive dyes or the drift associated with manual electrode handling. While classical TEER measurements do address these issues, conventional setups with the typical single frequency approach struggle to maintain good signal quality in microscale organ-on-chip configurations and altogether fail for cell types with low TEER response.
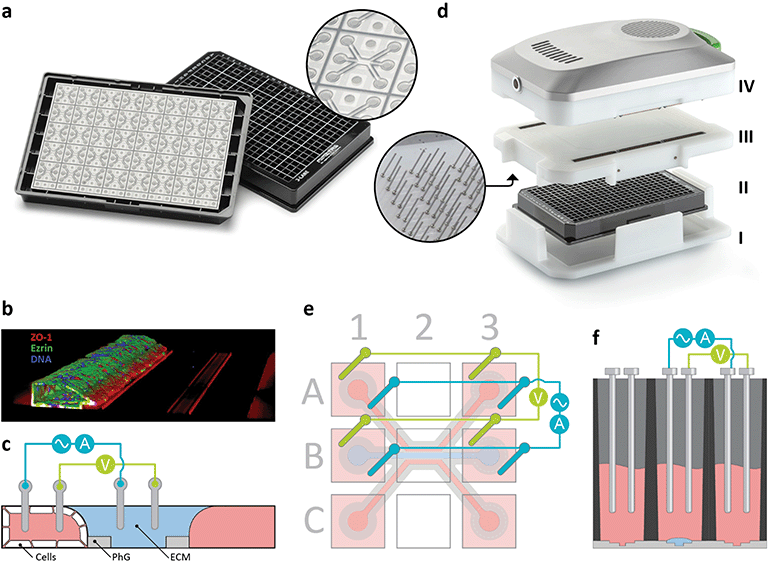
To support Mimetas’s goal of industrializing organ-on-chip readout, Sciospec engineered a custom complex impedance spectroscopy platform designed to resolve low-range signals in 3D tissues. Unlike single-frequency systems that fail to capture the nuances of epithelial tissue in modern miniaturized organ-on-chip models, this architecture sweeps up to 256 frequencies, allowing for the mathematical extraction of stable TEER values even in high offset, low-TEER environments.
Sciospec implemented a semi-parallel multichannel architecture accommodating 64 sensors and up to 768 electrodes per plate. This design enables the system to capture full-spectrum data for an entire plate in less than 30 seconds, facilitating dynamic assays that evolve faster than sequential measurement allows. The hardware was specifically engineered for low-thermal, incubator-ready operation, placing the measurement unit directly inside the controlled environment to prevent the temperature drift that typically compromises longitudinal data in perfused, membrane-free tissues.
Scaling this capability involved navigating constraints that extended well beyond the core engineering challenge. The partner faced significant pressure regarding validation timelines, organizational risk, and the seamless integration of new hardware into established workflows. To address this, Sciospec provided a turnkey OEM solution, developing not just the sensor hardware but also the complete software ecosystem required for high-speed data acquisition and analysis.
The operational hurdles and many unknowns for such a complex product development were addressed through a structured process of iterative alignment and system-level adaptation, rather than a simple technology handover. By defining and resolving these requirements jointly, the final platform emerged as a robust, fully integrated solution, significantly reducing the uncertainty and integration risk inherent in deploying novel technology.
Are you planning to move from research to routine application?
Mimetas leveraged this specific architecture to replace optical bottlenecks with a robust, label-free electronic readout.
Operational Impact: Eliminating bottlenecks and paving the way for scaling up
By shifting from optical endpoints to high-speed electrical monitoring, laboratories can fundamentally restructure their screening capacity. This architecture eliminates the need to block expensive optical readers for days, allowing multiple devices to operate simultaneously within standard incubators. This capability allows researchers to generate significantly more data per incubator shelf, reducing manual labor demands and enabling the parallelization required for pharmaceutical-grade pharmacological research and development.

The stability and throughput of this system have driven its adoption by major pharmaceutical entities, including Merck, Sanofi, and Roche, as well as leading academic research institutions worldwide. The platform is now a validated tool for critical applications ranging from intestinal toxicity to kidney-on-chip and blood-brain barrier models.
Dr. Philip Hewitt at Merck validated the platform’s role in their workflow:
The OrganoPlate® platform with the OrganoTEER® device will become a standardized assay in our early investigative toxicology drug screening workflow… the most suitable platform offering at the same time scalability, speed, robustness, ease of handling.
John Doe Tweet
Research published in Lab on a Chip (Nicolas et al., 2021) further confirms the technical superiority of this approach over traditional methods: “We show that TEER measurement is significantly more sensitive than a fluorescent reporter leakage assay… and capable of time-lapse monitoring under flow conditions.The instrument is revolutionary in terms of its throughput, ease-of-use, and capability to interrogate epithelia under flow and without interference of porous membranes.”
Mimetas leveraged this architecture to become a standardized assay. Let’s engineer the solution to scale yours.
Accurate measurement requires complex impedance spectroscopy rather than simple resistance checks. Because 3D tissues in organ-on-chip models often exhibit low absolute resistance, a system must sweep multiple frequencies (e.g., up to 256) to distinguish true epithelial barrier function from medium resistance and electrode interfaces. This spectral data is then processed using mathematical models to extract robust TEER values.
The ideal system for high-throughput screening must combine incubator-ready hardware with rapid readout capabilities to prevent thermal shock and maintain culture viability. It requires a cycle time of less than 30 seconds per plate and a semi-parallel architecture to capture fast transient signals. The OrganoTEER® is explicitly validated to meet these criteria, measuring up to 64 chips in under a minute without disturbing the culture environment.
Electrical TEER offers non-invasive monitoring that supports longitudinal studies, whereas optical methods often require destructive dyes or fluorescent reporters that can alter cell behavior. TEER provides a continuous, label-free sensing metric that tracks barrier formation and disruption over days or weeks, offering higher sensitivity to minute changes in barrier integrity compared to leakage assays.
The system utilizes a membrane-free electrode configuration compatible with microfluidic flow. By integrating microfluidic impedance sensors directly into the plate interface, the system measures transepithelial resistance under continuous perfusion. This allows for the assessment of cells in a physiologically relevant state, capturing the immediate impact of compounds or flow stress on the barrier.
Mimetas required <30 second readouts and complex impedance spectroscopy to industrialize their biology. What are the critical requirements for your instrument?

💡Curious for more? Check out our latest developments and products.
🧪Want to try it yourself? Contact us for more information
🚀Looking for a tailored solution? Our experts can help!
📢 Follow us on LinkedIn for more updates.
explore by relevant keywords
…or just browse our most recent posts: